Porometry: Analysis of through-pores in filters and membranes
The Capillary Flow Porometry is used for R&D and quality control in industries worldwide such as filtration, nonwovens, pharmaceutical, fuel cell, water purification and battery. Samples often tested include filter media, membranes paper, powders, ceramics, battery separators and health care products.
It measures the diameter of the most constricted part of a through pore (pore throat, largest pore diameter, Mean flow pore diameter, Pore diameter range, Pore distribution, Gas Permeability.
Analytical method
1. Capillary flow porometry
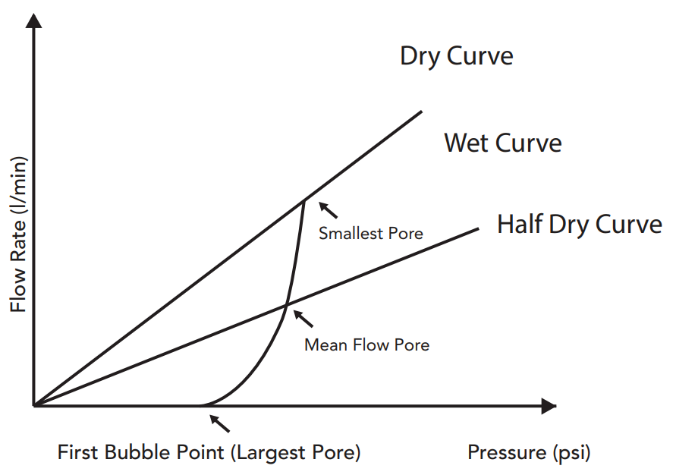
The standard capillary flow porometry is well known and very useful to characterize the pore structure of materials such as membranes, filter media, ceramics, paper, textile and similar materials. A non-toxic liquid is allowed to spontaneously fill the pores in the sample and a non-reacting gas is allowed to displace the liquid from pores by increasing the gas pressure. First the larger pores will get emptied, as the pressure increases more and more smaller pores are progressively emptied. The pressure and flow rate of gas through the emptied pores provides the through pore distribution and the first detectable flow pressure defines the so called bubble point, which is related to the maximum pore size in a sample.
Typically curve progression of a capillary flow porometry measurement
2. Liquid-liquid porometry (LLP)
However for many materials having very small pores (< 20nm) or for materials which doesn’t withstand relatively high pressures, liquid-liquid porometry (LLP) might be the better choice. It is a valuable technique for measuring the pore structure characteristics of ultrafiltration membranes. Such membranes can act as barriers to particles, including bacteria, pollens, spores, or pesticides. Further LLP is capable of measuring pore diameters, pore size distribution and liquid flow rates of materials having very low permeability. Typical examples are reverse osmosis membranes, nanofiltration membranes, blood purification membranes or battery separators. Very low liquid permeability is measured fully automated for pore diameters down to 3 nanometers and the needed pressures are much less than those for capillary flow porometer.
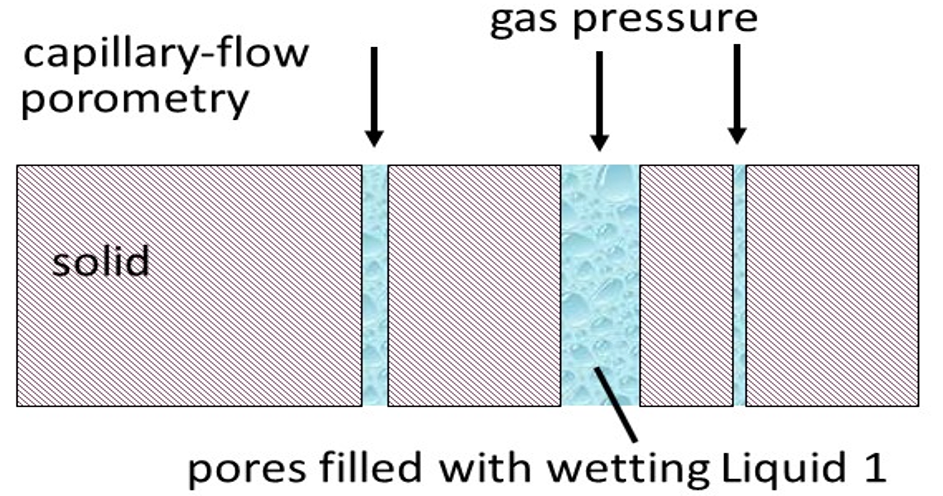
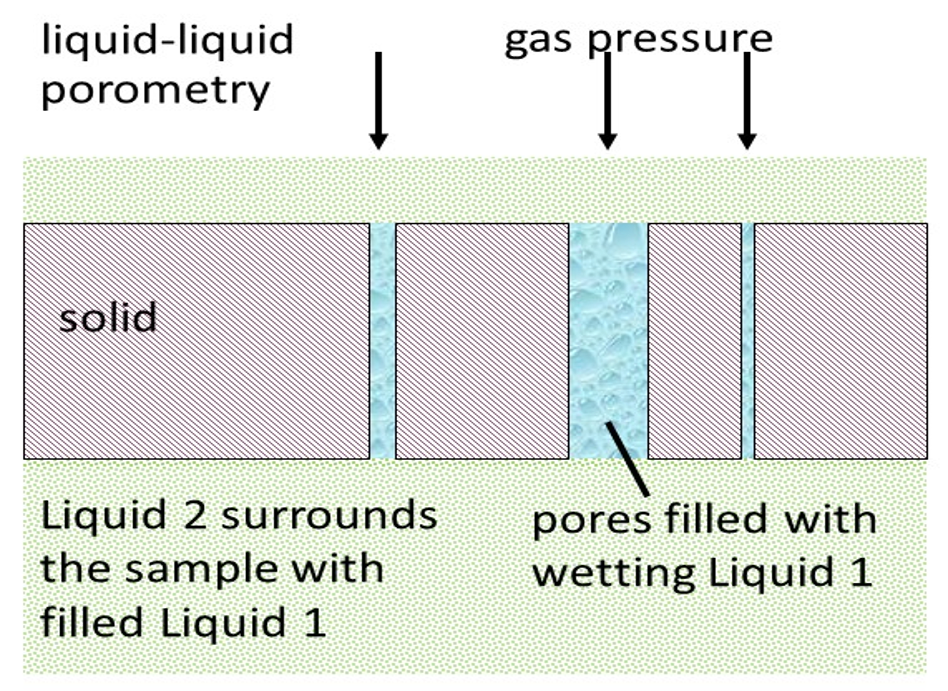
A wetting liquid spontaneously fills the pores of the material. Figure above shows the main difference between the conventional capillary flow porometry and the liquid-liquid porometry. In case of LLP, two immiscible wetting liquids are selected. Liquid 1 with lower surface tension is used to fill the pores of the sample. Liquid 2 is added to the top of the sample and is pressurized to displace the Liquid 1 from the pores and flow through the empty pores. The flow rate of Liquid 2 is also measured without wetting the sample with Liquid 1. The pore diameter is related to the surface tension of the two liquids. The flow rates yield pore distribution and liquid permeability. The working Equation for the LLP-method is:
D = 4 γ1 cos θ1 / p
with:
pore diameter → D
interfacial surface tension of liquids → γ1
contact angle of liquid 1 → θ1
A good choice for the Liquid-Liquid porometers from PMI is to take the immiscible and saturated wetting liquids such as silwick and alcohol. The pores of the material are filled with silwick as Liquid 1, and alcohol as Liquid 2 is pressurized to displace the silwick and flow through the pores. The amount of liquid flowing out is measured in balance. Alcohol flow rate and differential pressure are measured. Because surface tension of silwick and alcohol are low, contact angles are taken as zero. Mean flow pore diameter and pore distribution are computed like standard capillary flow techniques.
 Deutsch
Deutsch English
English