Measurement of the electrical conductivity in organic solvents
The conventional instruments are measuring the ohmic resistance of the sample to calculate the electrical conductivity. For this method, the lower limit of this parameter is about 0.5 µS/cm (electrical conductivity of destilled water).
The innovative conductivity probe DT-700 on the other hand enables the analysis of weak polar liquids like alcohols to non-polar solvents like toluene or n-hexane.
Instrument
Measurement principle
The DT-700 probe consists of two coaxial, cylindrical electrodes. During an experiment, a sinusoidal AC voltage is applied between the electrodes, and the electric current that flows between both is measured. The frequency of the applied voltage is adjusted automatically between 1 to 10 MHz independent of the measured conductivity. This experimental design enables the measurement of the electric current (and conductivity) over several decades. Finally, the specific electrical conductivity of the sample is calculated from the ratio of current to voltage and the cell constant of the probe. The cell constant is obtained by measuring three standards.
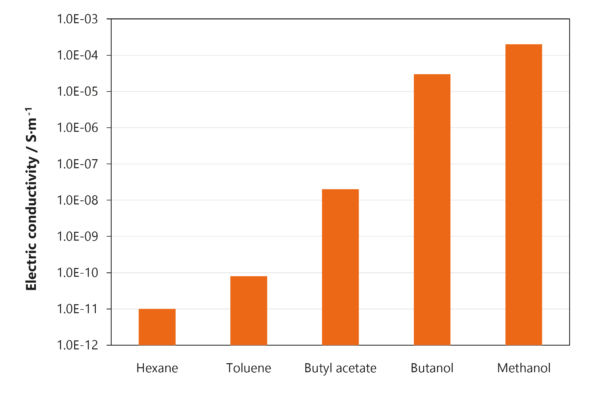
The figure shows exemplarily the repeated measurements of different liquids with electrical conductivities over the whole measurement range (from 10-11 S/m (n-hexane) to 10‑4 S/m (methanol).
 Deutsch
Deutsch English
English