Chemisorption and TPX
The chemisorption analysis is specially used to characterize catalysts. The most crucial point is to determine the chemically active part of the surface area. To determine the active surface area a measuring gas is used, which is able to strongly chemisorb at the active sites. Hydrogen gas is often used for the analysis, which creates a chemisorption bond to noble metals (e.g. platinum-alumina catalysts).
Physisorption vs Chemisorption
We are offering chemisorption analyses as contract analysis
1. Static-volumetric method
A certain amount of gas is dosed onto the active material in vacuum. The determination of the amount of adsorbed gas is carried out by pressure measurement in a system with known volume. Typically two isotherms are measured during the chemisorption experiment: The first isotherm reflects the sum of physisorption and chemisorption. After that the sample is evacuated to desorb loosely bonded gas molecules (physisorbed measuring gas) from the surface. Repeating the isotherm then only shows the physisorption again due to the blockage of the active sites from the first isotherm. Subtracting the second from the first isotherm only reveals the pure chemisorption. From this isotherm the amount of active sites can be calculated.
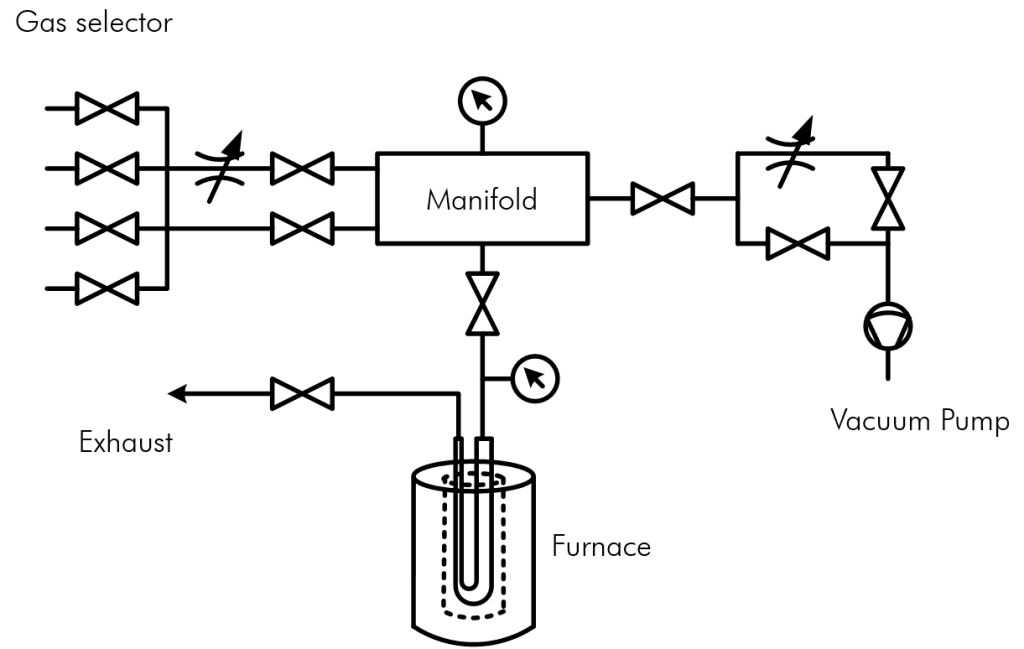
Experimental setup volumetric method
2. Dynamic method
2.1. Isotherm (Pulse chemisorption)
An inert gas continuously flows over a solid. A thermal conductivity detector analyses the measuring signal (base line). After that pulses of the measuring gas are added successively into the flow of the inert gas and after each pulse the instrument waits until the signal reaches the base line again. Initially the active material chemisorbs the measuring gas. After a while the actives sites are getting saturated. The measurement is being continued until the thermal conductivity detector shows constant peaks and no measuring gas is chemisorbed any more. This method is called pulse- or titration method.
Chemisorption experiments are often carried out temperature-controlled, please read more at measuring method „temperature-programmed reactions“.
2.2. Temperature-programmed reactions
Non-isothermal measurements are carried out usually through linear heating of a sample and continuous recording the changes of the gas composition. Temperature-controlled reactions can involve desorption (TPD), reduction (TPR), oxidation (TPO) and other relevant reactions for the characterization of catalysts.
Experimental setup dynamic method
Before performing temperature-controlled reactions to characterize catalysts the sample is prepared in-situ. Therefore so-called macros are defined, so that the procedure of the sample preparation is done fully automatically. The further approach is then task-oriented: A TPD experiment starts with the adsorption of active gas on a sample (e.g. by pulse chemisorption) followed by the characterization of the temperature-dependent desorption process. TPR reactions are done using a reducing gas, usually H2, TPO experiments are performed with an oxidizing gas, typically O2.
The experiments are carried out in a gas flow and changes in the gas composition are recorded by the thermal conductivity detector. Before performing an experiment, it has to be considered that not only the gas composition has to change during the reaction, also the thermal conductivity has to change due to ad- and desorbing molecules. In principle gases and vapors can be divided up into two main groups regarding their thermal conductivities:
1. H2, He
2. CO, CO2, Ar, NH3, H2O, Pyridin, N2O etc.
By this classification, the experiments can be easily derived. During a chemisorption reaction a gas/vapor from both group has to be present. Examples:
1. TPR with hydrogen (group 1) needs a carrier gas from group 2, e.g. Argon.
2. TPD of NH3 (group 2) requires a carrier gas from group 1, typically Helium.
3. TPO with Oxygen (group 2) needs a carrier gas from group 1, e.g. Helium.
The advantages of temperature-controlled reactions are not only to determine the active sites of catalysts but also determining on the different strengths of chemically active sites due to the temperature dependence.
 Deutsch
Deutsch English
English